Capacity building programme AMELIORER, in Benin
The clinical research context in Africa
With continued interest in advancing clinical trial conduct in sub-Saharan Africa, there is a growing imperative to strengthen pharmacovigilance systems, develop and reinforce the capacity of Ethics Committees, Regulatory bodies, and clinical research personnel in the region. Beyond the inception of any clinical trial, there is a requirement for effective pharmacovigilance systems to protect populations, highlight possible safety issues with new interventions, and help to build public trust in clinical research and health systems. The conduct of clinical research to the highest international standards requires approvals by competent authorities. Research Ethics Committees (RECs) play a pivotal role in this process, responsible for protecting the safety and welfare of humans as research participants, by providing an independent evaluation of proposed research and ensuring that it is planned and conducted in accordance with the applicable ethical principles, standards, and regulations in a timely manner. The complexity of research designs has increased over the past two decades, creating demand for deeper technical expertise of REC members to review complex and emerging study designs. Lack of expertise has been cited as one of the barriers hindering conduct of clinical trials in developing countries. Chapman et al highlighted that many RECs struggle with evaluating and providing oversight for unusual study designs such as innovative phase I trials due to a lack of technical competence when conducting reviews. Moreover, the schedule for review is rarely achieved by the RECs. This is concerning for researchers whose research is often timebound and who have obligations to meet funder timelines and budget restrictions.
Due to a lack of physical infrastructure, inadequately trained personnel, and weak institutions in countries with high disease burdens, it has proven difficult to develop a strong track clinical research record to compete successfully for international funding.
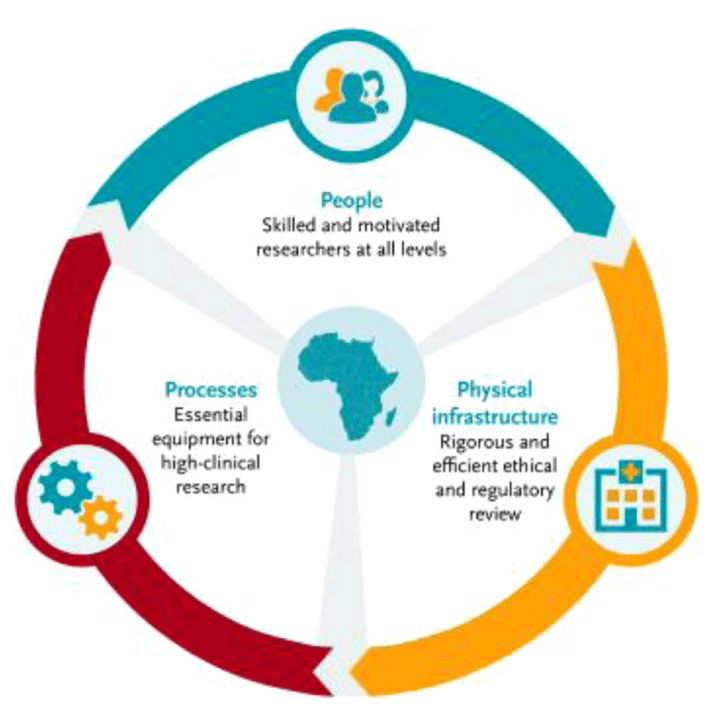
EDCTP (European & Developing Countries Clinical Trials Partnership) capacity development integrated approach. From Thomas Nyirenda T. et al. Strengthening capacity for clinical research in sub-Saharan Africa: partnerships and networks. International Journal of Infectious Diseases 110 (2021) 54–61.
What has become clear is that strengthening research capacity in Africa is critical to enhancing Africa’s self-sufficiency, preparedness for the next public health emergency, and ability to fight against the endemic infectious diseases.
Pharmalys knows how to deliver capacity building
The Pharmalys team has conceived and delivered seven capacity building programmes to Ethics Committees and Regulatory Authorities throughout Africa so far; ERUDIT in Togo, ERECIS in eSwatini, BRECOR in Rwanda, STREC in Ghana, AMELIORER in Benin, CECaBI in Côte d’Ivoire, and ERC in The Gambia. Read more: Capacity Building | Pharmalys
One of Pharmalys’ most recent programmes was Ethics and Regulatory Capacity (ERC) in The Gambia. Health research is a major pillar in the development of the health sector in this country. Aligned with the Commission on Health Research for Development (COHRED), which states that ‘building research capacity in developing countries is one of the most powerful, cost-effective and sustainable ways of advancing health and development’, a consortium of different partners, named Ethics and Regulatory Capacity in The Gambia (ERC The Gambia), collaborates to transform the ethical and regulatory practices in the country.
As part of this consortium, Pharmalys is responsible for providing advanced training in Research Ethics, Good Clinical Practice, Clinical Trial coordination and Trial field inspection. This series of highly targeted training courses aim to equip senior members of the National Ethics Committee for Life Sciences and Health authorities with the knowledge and skills they need to conduct high quality ethics reviews, ensure optimal protection of research participants, and effectively manage and coordinate clinical trials.
To achieve these objectives, Dr. Karim Bagaté, Director of Clinical Operations & Safety and Mr. Assane Ndiaye, C.R.A. Manager at Pharmalys, provided a one-week intensive training course on Good Clinical Practice (GCP) and performed a clinical trial inspection, to ensure the staff were working in accordance with the protocol and GCP guidelines.
The training covered many topics from the drug development process and history of ICH GCP, to fraud, misconduct & research integrity, including the management of the investigational product, drug safety, essential documents and quality assurance to name but a few. Learn more: Quality Assurance | Pharmalys.
The 2-day clinical trial site mock inspection consisted in a document review to check the availability, completeness, authenticity and GCP compliance of the documents, interviews with the investigational team to assess the site organisation and the team training, and a site visit to evaluate the organisation of the various sites. Learn more: www.pharmalys.com
Conclusion
All funded studies must meet acceptable international scientific, ethics, and regulatory standards for the conduct of clinical trials and implementation research. To achieve excellence in collaborations, study design, and conduct that meet international standards, one key objective is to build local capacity where the research is conducted.
Africa is a continent with a relatively youthful demographic. Given the unique challenges posed by a high prevalence of infectious diseases within this demographic, there is a critical necessity to build capacity for clinical research. The cultivation of local expertise and resources is not only pivotal for the successful realisation of high quality research initiatives, but for addressing the specific health concerns within the African continent.
This article was written from the following references:
Ainembabazi P. et al. J Empir Res Hum Res Ethics. 2023 July ; 18(3): 91–98.
Chapman A.R. Addressing the Ethical Challenges of First-in-Human Trials. Journal of Clinical Research Bioethics 2011; 2(4). doi:DOI: 10.4172/2155-9627.1000113.
Lawal Y. Africa’s low COVID-19 mortality rate: A paradox? Int J Infect Dis 2021;102:118–22.
Nyirenda T. et al. Strengthening capacity for clinical research in sub-Saharan Africa: partnerships and networks. International Journal of Infectious Diseases 110 (2021) 54–61.