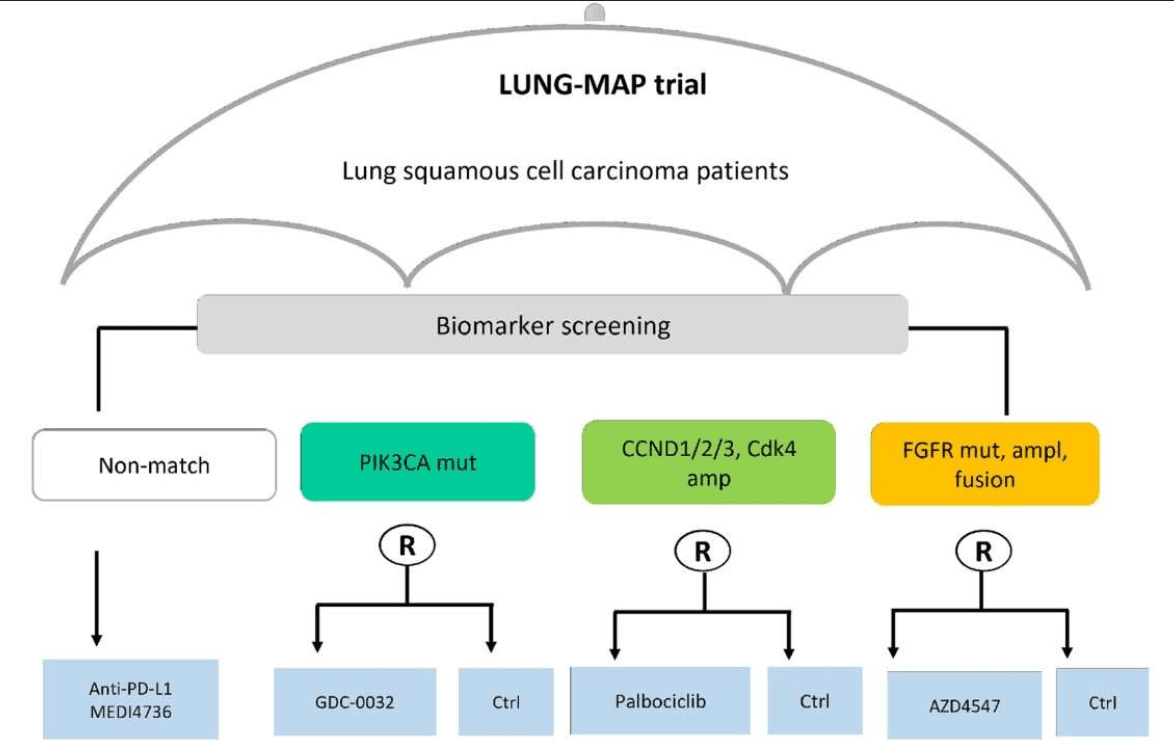
Example of a real umbrella trial. Study scheme of the lung cancer master protocol (Lung-MAP–NCT03851445) trial. From Ouma L.O. et al. (2022) Front. Med. 9:1037439. doi: 10.3389/fmed.2022.1037439
In recent decades, cancer treatment has evolved from relying on non-specific, cytotoxic chemotherapy and radiation to adopting a more targeted approach, driven by advancements in omics technology. As these technologies have become more sophisticated and widely accessible, it’s now possible to tailor treatments to the molecular abnormalities present in each patients’ tumour. Genomic studies have revealed that individual tumours, particularly metastatic ones, exhibit significant complexity and heterogeneity. This means that the most effective therapies must be customised to the individual, fueling the growing interest in precision medicine. Precision medicine aims to enhance treatment outcomes by identifying therapies specifically tailored to the genetic makeup of a patient’s disease. (ie, targeted therapies).
In the United States, momentum toward precision medicine has grown significantly. In May 2018, the National Institutes of Health launched their “All of Us” Initiative, aiming to collect demographic and biological data from over one million people to improve precision care in oncology and other medical fields. Similarly, in September 2018, the US Food and Drug Administration (FDA) released draft guidance for master protocols, signalling their support for wider adoption of precision-based research methods. In the United Kingdom, the National Health Service’s 2019 Long-Term Plan highlights precision oncology as a key area of focus.
The rising interest in precision care underscores the importance of biomarkers in the development of targeted therapies. One of the most notable methodological advancements in biomarker-guided clinical trials has been the development of master protocols, which are overarching trial designs that evaluate multiple hypotheses. These protocols aim to improve efficiency by standardising trial procedures. Two primary types of master protocols are basket and umbrella trials.
Introduction to Basket and Umbrella Trials
Basket trials are prospective clinical studies that test one or more targeted treatments across multiple types of diseases. These trials use unifying eligibility criteria that group patients with different diseases, such as multi-histological cancers, into a single trial. The common factor for inclusion is usually a predictive biomarker associated with the intervention’s mechanism of action, as it helps determine whether a patient is likely to respond to the treatment.
For example, a phase 2 basket trial conducted by Li et al. evaluated whether ado-trastuzumab emtasine, an FDA-approved drug for HER2-positive (Human Epidermal growth factor Receptor 2) metastatic breast cancer, could induce an antitumour response in HER2-amplified or HER2-mutant cancers across multiple histologies (https://pubmed.ncbi.nlm.nih.gov/29989854/). Patients with advanced lung, endometrial, salivary gland, biliary tract, ovarian, bladder, colorectal, and other cancers were included based on HER2 amplification or mutation as the common eligibility criterion. Traditionally, early-phase cancer clinical trials often included patients with various histopathologies to assess treatment signals. However, basket trials, which take a histology-agnostic approach, are now being considered for phase 2 and even some phase 3 evaluations.
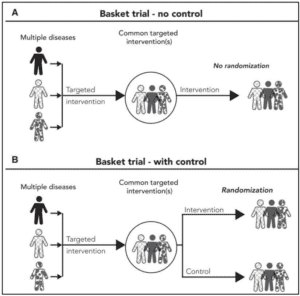
Illustrative examples of a basket trial. (A) A single-arm basket trial with a single targeted intervention without a control group is illustrated. (B) A 2-arm randomized basket trial is shown. From Park J.J.H. et al. CA CANCER J CLIN 2020;70:125–137.
Umbrella trials, in contrast, focus on a single disease and test multiple targeted therapies based on predictive biomarkers or other predictive patient risk factors. These trials stratify a disease, such as advanced breast cancer, into several subgroups. Each subgroup received a treatment tailored to the molecular characteristics of their disease.
One example is the plasmaMATCH umbrella trial, which tested five therapies for advanced breast cancer. The therapies were divided into five treatment groups based on patients’ molecular signatures (clinicaltrials.gov identifier NCT03182634). Patients in group A had breast cancer with an ESR1 (estrogen receptor gene 1) mutation, they received an extended dose of the estrogen receptor downregulator fulvestrant. In group B, patients had an HER2 mutation and received an HER tyrosine kinase inhibitor and fulvestrant if they had an estrogen receptor co-mutation. Group C patients had an AKT (serine/threonine-specific protein kinase B) mutation and received the AKT inhibitor AZD5364 plus fulvestrant. Group D patients had AKT activation and received AZD5364 only, and group E patients with triple-negative breast cancer received the poly(ADP-ribose) polymerase inhibitor olaparib plus AZD5364. In this umbrella trial, multiple biomarker essays were applied to a single tumour histology, and patients were assigned to 1 of the 5 subgroups based on their biomarker status to evaluate the clinical utility of 5 different targeted therapy strategies for advanced breast cancer.
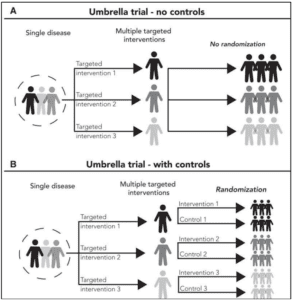
Illustrative examples of an umbrella trial. (A) A nonrandomized umbrella trial with 3 targeted interventions is illustrated. (B) A randomized umbrella trial that includes 3 subgroups, each with a targeted intervention and a control group. From Park J.J.H. et al. CA CANCER J CLIN 2020;70:125–137.
Conceptually, an umbrella design is simply a set of (sub)trials run in parallel. It offers a selection of appealing advantages, including: (i) that several treatment-related questions can be answered in a single trial, (ii) a potential reduction in the number of patients required (for instance, by including a common control arm), and (iii) expedited drug development, with shorter trial duration and lower costs overall, relative to running traditional clinical trials independently. However, numerous statistical complexities may arise in the conduct of umbrella trials, including but not limited to a desire for adaptive design elements, the choice between Bayesian/frequentist decision rules, appropriate sample size calculation, whether to borrow information, and how to control particular error rates.
Key Characteristics of Basket and Umbrella Trials
There are important distinctions between basket and umbrella trials in terms of their eligibility criteria, patient subgrouping, and intervention assignment. From Park J.J.H. et al. CA CANCER J CLIN 2020;70:125–137.
Eligibility criteria:
- Patients enrolled in a basket trial have multiple diseases with common unifying risk factor(s)
- Patients enrolled in an umbrella trial usually have the same disease
Patient subgroups:
- Basket trials: Patient subgroups may be defined based on disease subtypes
- Umbrella trials: Risk factors are used to stratify patients into multiple subgroups (patient stratification)
Intervention assignment:
- Basket trials:
- It is common for basket trials to have a single intervention that is targeted based on the unifying risk factor
- Intervention assignment may or may not be determined using randomisation
- Umbrella trials:
- Umbrella trials have multiple interventions, with intervention assignment being determined based on their risk factor
- Similar to basket trials, intervention assignment may or may not be determined using randomisation
Choice in a control group:
- Basket trials:
- Determining the choice in the control group can be difficult because there are multiple diseases being studied
- If there are different established standards of care between multiple diseases being studied, a common control group may not be feasible
- Umbrella trials:
- Compared with basket trials, it may be easier to pick the choice in the control group for umbrella trials because there is one disease being studied
- The existing standard of care (or placebo, if there is no established care) for the disease being studied may be used as the control for all of the subgroups
Conclusion
Most basket and umbrella trials have been conducted in oncology, with many led by investigators from the US National Cancer Institute (NCI), industry sponsors, and contract research organisations. Despite their growing number, these trial designs are still not widely understood, making it essential to improve the general knowledge and literacy of these approaches.
Precision medicine is revolutionising the way clinical trials are conducted. Future trials need to focus on matching treatments to patients based on their tumour biomarkers rather than matching patients to specific trials. Given the high cost, vast resources, and complexity of conducting traditional clinical trials, master protocols offer a more efficient and effective way to evaluate targeted therapies across small patient subpopulations.
Veronique Ropion, MD
Strategic Projects Director, Pharmalys Ltd
Bibliography:
- Chen A.P. et al. National Cancer Institute Basket/Umbrella Clinical Trials: MATCH, LungMAP, and BeyondCancer J. 2019 ; 25(4): 272–281. doi:10.1097/PPO.0000000000000389
- Fountzilas E. et al. Clinical trial design in the era of precision medicine. Genome Medicine (2022) 14:101-128.
- Ouma L.O., Wason J.M.S., Zheng H., Wilson N., Grayling M. (2022) Design and analysis of umbrella trials: Where do we stand? Front. Med. 9:1037439. doi: 10.3389/fmed.2022.1037439
- Park J.J.H. et al. An Overview of Precision Oncology Basket and Umbrella Trials for Clinicians. CA CANCER J CLIN 2020;70:125–137